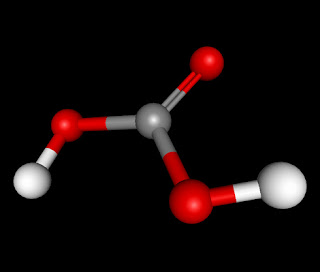
The molecule H2CO3 (also known Carbonic Acid) is an AX3, triangular planar, molecule. It has numerous intermolecular forces acting upon it when it bonds with another molecule of Carbonic Acid. The forces which act on H2CO3 are London Dispersion Forces, Dipole-Dipole, and Hydrogen Bonding. It has London Dispersion Forces acting upon it because they exist between any and every molecule and it is the weakest of the forces that it has. The next force that acts upon it is Dipole-Dipole. This is because it is a polar molecule, the double bonded oxygen side being negative and the side with two the two hydrogen attached to the oxygen being positive. The last and strongest intermolecular force in H2CO3 is Hydrogen Bonding. This would happen between one of the two Hydrogen molecules and any of the three Oxygen molecules.
ATTENTION!!! LIMITED TIME OFFER!!!!
Do you like soda? The refreshing taste of the bubbly liquid trickles down your throat on a hot summer's day? The fizzy tingle as the soda hits your lips quenching your thirst? Do you hate flat soda? Then H2CO3 is for you!!! Carbonic Acid is what is used to carbonate most carbonated beverages. When soda is being made, carbon is dissolved in water, and thus Carbonic Acid is formed. This is what also gives the carbonated beverages the distinct tart taste that we all have come to love. It makes the fizzy drinks taste fizzy.
Another great reason to try some H2CO3 is if you like to be able to be active and live a health life, and well, who doesn't like that? Carbonic Acid has an important role in our body to help keep our pH level stable. If the pH changes, whether up or down, enzymes can stop functioning, muscles and nerves can weaken and your metabolic activities can become impaired. Luckily Carbonic Acid releases bicarbonate ions which can act as an acids or a bases as need be and therefor ensuring us to be in peak physical condition.
So if you like to drink carbonated beverages or like being healthy and able to be active then buy H2CO3 today!