The molecule H2CO3 (also known Carbonic Acid) is an AX3, triangular planar, molecule. It has numerous intermolecular forces acting upon it when it bonds with another molecule of Carbonic Acid. The forces which act on H2CO3 are London Dispersion Forces, Dipole-Dipole, and Hydrogen Bonding. It has London Dispersion Forces acting upon it because they exist between any and every molecule and it is the weakest of the forces that it has. The next force that acts upon it is Dipole-Dipole. This is because it is a polar molecule, the double bonded oxygen side being negative and the side with two the two hydrogen attached to the oxygen being positive. The last and strongest intermolecular force in H2CO3 is Hydrogen Bonding. This would happen between one of the two Hydrogen molecules and any of the three Oxygen molecules.
ATTENTION!!! LIMITED TIME OFFER!!!!
Do you like soda? The refreshing taste of the bubbly liquid trickles down your throat on a hot summer's day? The fizzy tingle as the soda hits your lips quenching your thirst? Do you hate flat soda? Then H2CO3 is for you!!! Carbonic Acid is what is used to carbonate most carbonated beverages. When soda is being made, carbon is dissolved in water, and thus Carbonic Acid is formed. This is what also gives the carbonated beverages the distinct tart taste that we all have come to love. It makes the fizzy drinks taste fizzy.
Another great reason to try some H2CO3 is if you like to be able to be active and live a health life, and well, who doesn't like that? Carbonic Acid has an important role in our body to help keep our pH level stable. If the pH changes, whether up or down, enzymes can stop functioning, muscles and nerves can weaken and your metabolic activities can become impaired. Luckily Carbonic Acid releases bicarbonate ions which can act as an acids or a bases as need be and therefor ensuring us to be in peak physical condition.
So if you like to drink carbonated beverages or like being healthy and able to be active then buy H2CO3 today!
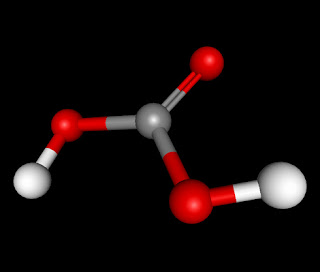

Your ad is very persuasive. You correctly determined if it was polar. You correctly determined the bonding. The model is correct. The overall design of your page is dark which directs your attention to the content.
ReplyDeleteI like the black on black background. It lights up the words and makes it easier to read. You did a really good job explaining the three forces of attraction and how they apply to Carbonic Acid. The 3D model is cool looking and you can tell where the positive side is and where the negative side is. You correctly determined that the molecule is polar. I really like the ad, and it makes me like Carbonic Acid. Good job Ben Long
ReplyDeleteYou're Ad is very Nice I like how it relates to what we drink everyday. The black background draws attention to the Text and images, well done with that. You're 3-D model is correctly shown with the correct angles. And you determined the polarity correctly, the molecule is polar. Very well Done.
ReplyDeleteThis blog is very useful because it gets straight to the point. The facts seem organized and you included a correct 3-D representation of Carbonic Acid. The angles are coorect and you mentioned correctly that it was polar.
ReplyDelete1.The overall design of your page is dark which directs your attention to the content.
ReplyDelete2. All of the diagrams/models are correct.
3. You correctly determined that it was polar.
4. You correctly determined the intermolecular forces and the bonding.
5. Your ad is very persuasive.